

Featured in Nature Synthesis, a new breakthrough electrolysis process that’s nearly 6 times more efficient at producing hydrogen, is discovered by a scientific team that includes Shanghai Dynamic Hydrogen, a CM portfolio startup
Nature Synthesis feature:
https://www.nature.com/articles/s44160-023-00444-x
With hydrogen energy – a clean energy that produces only water as a byproduct – becoming increasingly part of our world, the industry is constantly looking for efficient ways to produce hydrogen. Water electrolysis is the most common way as it splits H2 from O, but the process is limited by the Oxygen Evolution Reaction (OER), a key (but slow) chemical process which oxygen and hydrogen are separated. It’s known to be a major bottleneck.
To enable the electrolysis process, key precious metals are required to activate the reaction: usually platinum, iridium or ruthenium.

Idrium (Ir) and ruthenium (Ru) are rare earth metals, but iridium (500 USD/ounce) is exceptionally rare, with a production rate of just about 9 tons a year, mostly from South Africa. It’s more commonly found on meteorites, and is 1 of 9 least abundant stable elements in the Earth’s crust. As a reference, gold is 40 times more abundant than iridium.
Between the two metals, ruthenium (42 USD/ounce) is more common and costs less, making it more accessible, which also means helps to make hydrogen more accessible.
However, Ruthenium possesses superior catalytic activity to iridium but lacks stability in the challenging conditions of a PEM electrolyzer stack (for hydrogen production).
Dr Fan Lv and Guo Shaojun, from Shanghai Dynamic Hydrogen, a CM portfolio startup, were part of a scientific team that has discovered a way to stabilize Ruthenium (RU) in the electrolysis process in producing hydrogen.
Nature Paper excerpt:
Water electrolysis powered by renewable electricity can produce clean hydrogen, but the technology is limited by the slow anodic oxygen evolution reaction (OER). The most active monometallic OER catalyst
is high-valence ruthenium, but it is thermodynamically unstable. Here we leverage the strong and tunable interaction between substrate and active site found in single atom catalysts, and discover a local electronic manipulation strategy for stabilizing high-valence Ru single sites (Ru SS) on a class of Ni-based phosphate porous hollow spheres (Ru SS MNiPi PHSs where M = Fe, Co, Mn, Cu) for efficient electrolysis. Both X-ray absorption fine structure and density functional theory calculation results verify the intrinsic stability of the catalyst, and suggest that this originates from the tailored valence state, coordination number and local electronic structure of the Ru SS. We formulate general guidelines for stabilizing high-valence catalytic sites and introduce a double-volcano plot to describe the superior electrocatalytic behaviours of high-valence Ru SS. The optimum Ru SS/FeNiPi achieves a low overpotential of 204 mV and 49 mV for the OER and hydrogen evolution reaction at 10 mA cm−2, respectively. Assembling Ru SS/FeNiPi in an industrial-level electrolyser with a low Ru loading of 0.081 mg cm−2 realizes a stable industrial current density of 2,000 mA cm−2 at 1.78 V, which is the highest reported value in alkaline electrolyte to the best of our knowledge, and exceeds that of commercial Pt//RuO2 by 5.7 times.
The excellent stability of Ru SS/FeNiPi PHSs was investigated at an industrial-level current density of 500-4,000mA cm-2 (Fig 2g). No dramatic change of the potential was observed, further confirming the potential of Ru SS/FeNi PHSs for large-scale hydrogen production via water electrolysis.
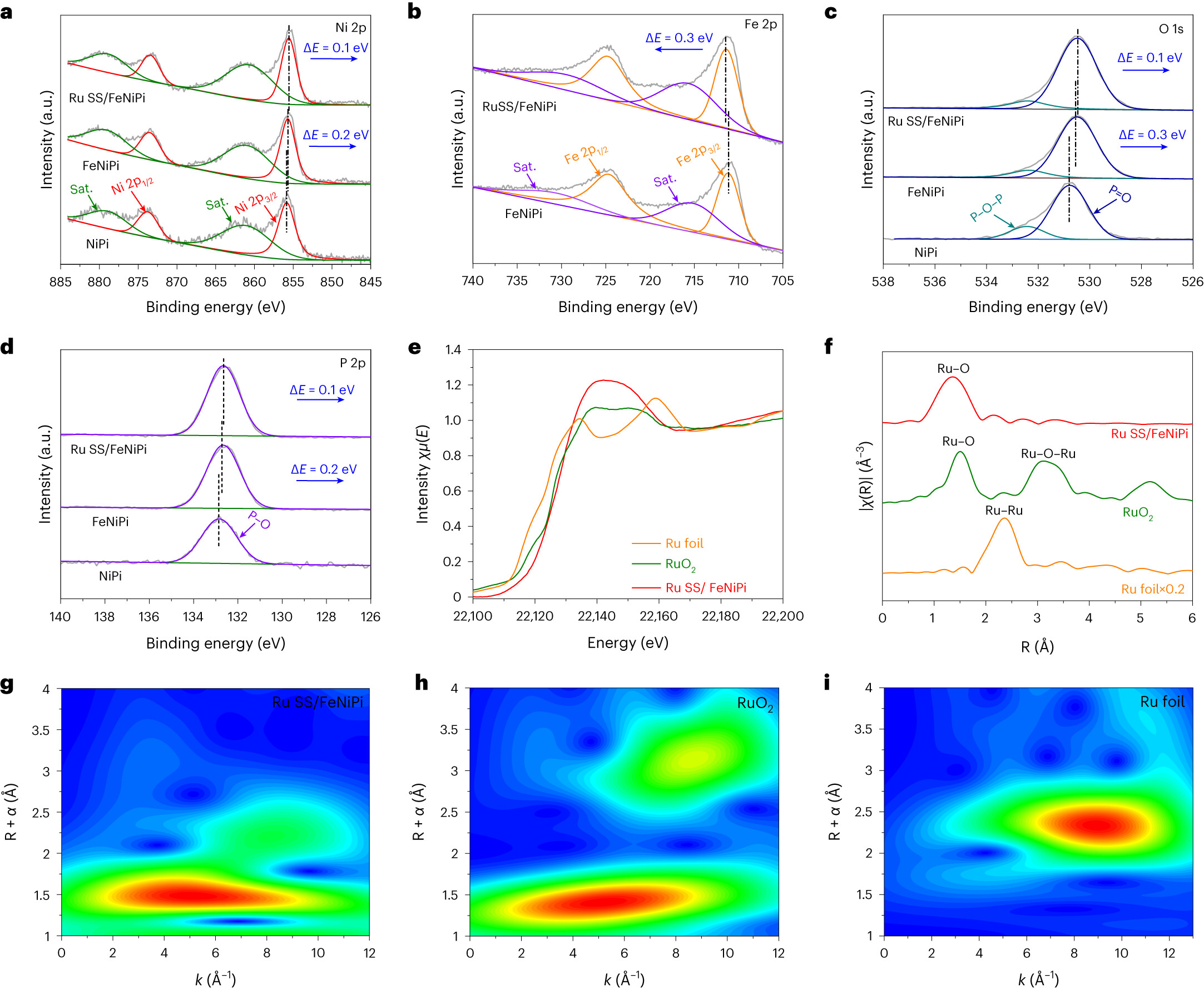
Fig. 3 | Electronic structure characterization of Ru SS/FeNiPi PHSs. a, Ni 2p XPS spectra of the Ni phosphate (NiPi), FeNi phosphate (FeNiPi) and Ru SS/FeNiPi PHSs. b, Fe 2p XPS spectra of the FeNiPi and Ru SS/FeNiPi PHSs. c,d, O 1s (c) and P 2p (d) XPS spectra of the NiPi, FeNiPi and Ru SS/FeNiPi PHSs. e,f, The XANES spectra (e) and Fourier-transformed Ru K-edge EXAFS spectra (f) of Ru SS/FeNiPi PHSs, RuO2 and Ru foil. g–i, The WT EXAFS signals of Ru SS/FeNiPi PHSs (g), RuO2 (h) and Ru foil (i).
The result is an industrial-level electrolyzer system with a highest reported alkaline electrolyte output value – to the best of our knowledge – that exceeds traditional platinum-ruthenium-based systems by 5.7 times.
Also, Ruthenium-based PEM systems to produce hydrogen are gaining popularity. Heraeus – a CM Venture LP – together with Sibanye-Stillwater, with just unveiled a ruthenium-based catalyst for PEM electrolysis to produce hydrogen.